
Image-guided therapy
Fiber Optic RealShape (FORS) technology
Using light instead of X-ray
Using light, Fiber Optic RealShape (FORS) technology, exclusive to Philips, enables real-time, 3D visualization of devices1 inside the body without the need for fluoro! FORS-enabled guidewires have a hair-thin optical fiber integrated into them. By pulsing light into the fiber and analyzing the reflections, the full shape of devices can be reconstructed and visualized. These devices are displayed in the context of the patient’s anatomy through overlay on images acquired before or during the procedure, like CT scans and X-rays.
LumiGuide
Turning light into data with 3D device guidance in a radiation-free environment
LumiGuide – powered by Fiber Optic Real Shape – gives you unprecedented, full-shape 3D device1 visualization. From any angle. In real-time. With distinctive colors. And integrated into your known roadmap techniques.
Device visualization using the wonders of light
With FORS technology, clinicians can carry out parts of minimally invasive interventions without the need for X- ray, which should minimize the risks associated with long-term radiation exposure.

Unprecedented viewing clarity
FORS technology makes it possible to display the full shape of devices in 3D and in distinctive colors, as opposed to the 2D, greyscale visualization produced by fluoroscopy. FORS images are generated in real-time, and can be rotated and tilted to allow viewing from any angle.
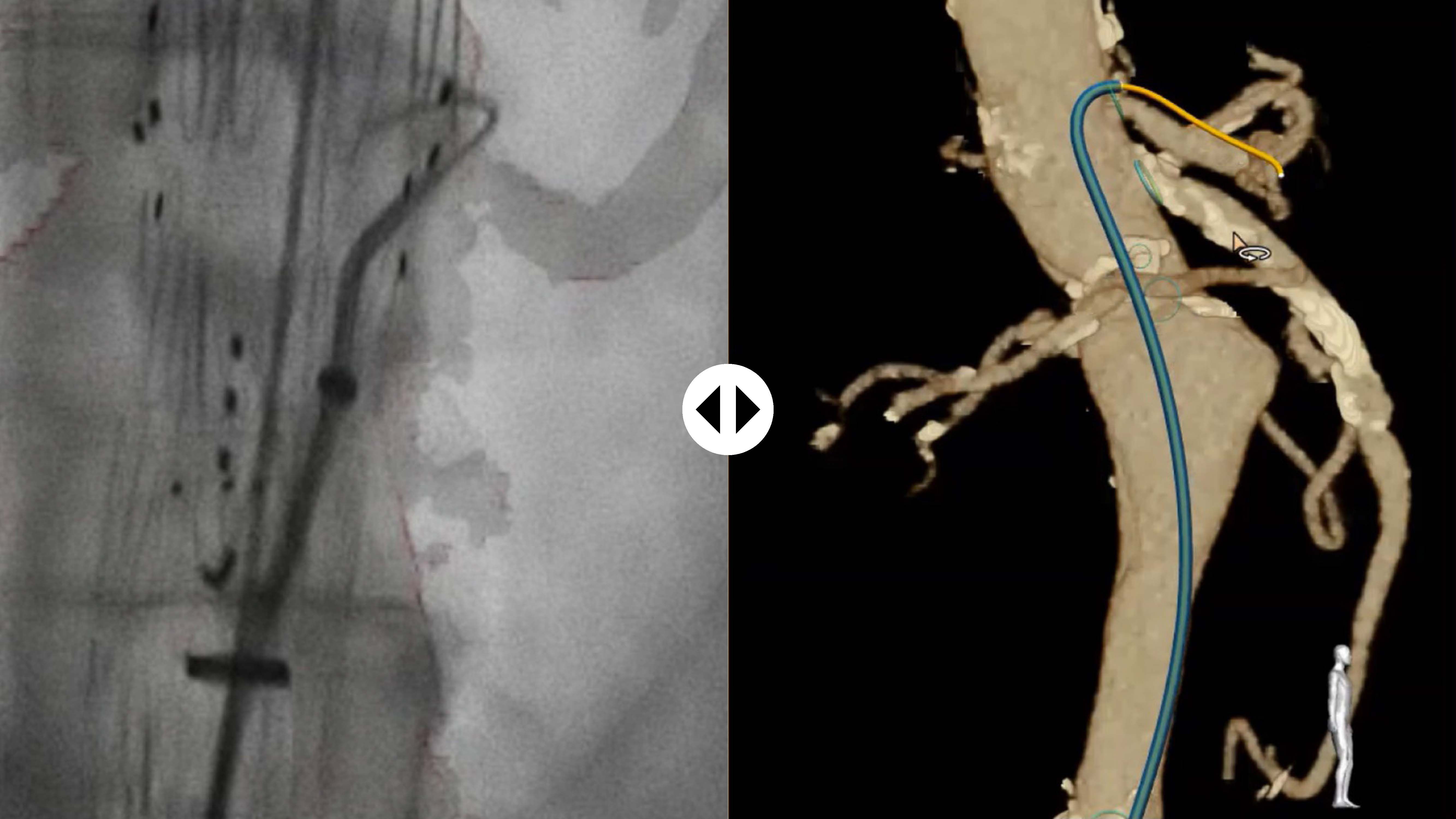
Visualize your catheter of choice
Thanks to 3D Hub technology, you can continue to work with the catheters you’re already used to. The 3D Hub, in combination with FORS-enabled guidewires, enables your catheters to be visualized inside the body.

Discover Philips LumiGuide powered by FORS technology
Work confidently and safely in an increasingly radiation-free lab. With LumiGuide, you see more, perform fast, stay safe – and push the boundaries of care for your patients.

Frequently asked questions
Documentation
First-in-Human Clinical Feasibility Study of Endovascular Navigation with FORS Technology
PDF|(1.28 MB)
Initial single-center experience with Fiber Optic RealShape guidance in complex aortic repair
PDF|(832.85 KB)
The effect of FORS-technology on the reduction of radiation during complex endovascular surgery
PDF|(541.64 KB)
Footnotes
- The FORS-enabled guidewire as well as compatible endovascular catheters.
- Jansen, van Herwaarden, et al. Three Dimensional Visualisation of Endovascular Guidewires and Catheters Based on Laser Light instead of Fluoroscopy with Fiber Optic RealShape Technology: Preclinical Results. European Journal of Vascular & Endovascular Surgery, April 2020. DOI: 10.1016/j.ejvs.2020.02.035
- Megens, 't Hooft, et al. The technology achieves submillimeter precision and provides full three-dimensional shape, surpassing the reported precision of other navigation and tracking technologies. Medical Physics, April 2021. DOI: 10.1002/mp.14881
- Bydlon, Flexman, et al. Visualization of navigation catheters is highly accurate with the 3D Hub technology. EJVES Vascular Forum, May 2023. DOI: 10.1016/j.ejvsvf.2023.05.006
Disclaimer
LumiGuide Equipment is FDA – 510(k) cleared and pending CE marking. LumiGuide Single-Use Devices are FDA – 510(k) cleared and CE approved.